- Lewis Dot Structure Calculator With Dots
- Covalent Lewis Dot Structure
- Covalent Lewis Dot Structure Calculator Answer

Chemical bond polarity is the concept that explains the property of sharing an electron between two elements. Covalent bond between the elements can be either polar or non-polar. This is determined with the concept of electro-negativity. If the electrons are shared equally between the atoms then its a non-polar covalent bond. If one of the atom is electronegative, it has more tendency to attract the electrons. Then the bond is called as polar covalent bond. This calculator is used to find the bond polarity and tendency of electro-negativity in each element.
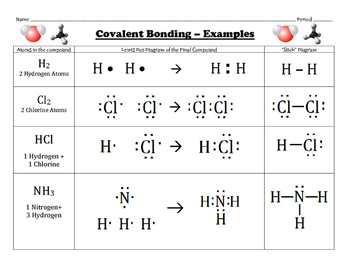
Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. There is a negative sign on the species, so we have an extra electron to consider. Following the rules for Lewis electron dot diagrams for compounds gives us. B has 3, each F has 7, and there is one extra electron: 3 + 7 + 7 + 7 + 7 + 1 = 32. In this lesson students learn how to write Lewis Structures for covalent compounds. This builds on their prior knowledge of how to write Lewis Structures for Ionic Compounds from Unit 3 Lesson 4 and students knowledge of Covalent Compounds from Unit 3 Lesson 6. This lesson aligns with NGSS Performance Expectation: HS-PS1-2: Construct and revise an explanation for the outcome of a simple. Lewis Dot Structures can be used to show how covalent compounds share electrons between atoms. Plan your 60-minute lesson in Science or Chemistry with helpful tips from Rachel Meisner. This is a good Lewis electron dot diagram for BF4−. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Food and Drink App: Vitamins and Minerals. Illustrate covalent bond formation with Lewis. Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. How the molecule might react with other molecules. The physical properties of the molecule (like boiling point, surface tension, etc.).
Lewis Dot Structure Calculator With Dots

Calculator of Chemical Bond

Chemical Bond Polarity

Covalent Lewis Dot Structure
Nonpolar Covalent:
If, Electronegativity Difference = 0
Polar Covalent:
If, 2 > Electronegativity Difference > 0
Covalent Lewis Dot Structure Calculator Answer
Ionic (Non-Covalent):
If, Electronegativity Difference > = 2